remdesivir and cirrhosis
remdesivir and cirrhosis
· Remdesivir particularly improves the survival outcomes of patients under oxygen support compared with those of patients receiving placebo 3 However clinical trials on remdesivir treatment excluded patients with hepatic impairment 3–6 Currently little is known about the experience of remdesivir administration in COVID-19 patients with severe hepatic cirrhosis,
Author : Takumi Umemura Kazuki Nishikawa Yoshikazu Mutoh, Hajime Sasano, Koji Kozaki, Tetsuya Yamada, Toshi
COVID-19 in patients with cirrhosis: understanding adverse
· Management of Remdesivir-Associated Acute Liver Failure Examined in Case Series Remdesivir was immediately discontinued in both patients …
Criteria for Use: Remdesivir
· Fichier PDF
Cirrhosis AST and/or ALT > 5 times the normal b Initiation after 24 hours of mechanical ventilation c Patients with life-expectancy less than 6 months 3 Duration of Therapy a 5 days of therapy upon initiation i Select patients originally set to receive 5 days of therapy but are subsequently intubated after initiation may be extended to 10 days b, Length of acute care hospital stay
High rates of 30-day mortality in patients with cirrhosis
Remdesivir
cirrhosis and a recent review did not mention any details on this topic 9 Besides, thisisthe firstreport to the best ofourknowledge of remdesivir administration in a COVID-19 patient with severe
Patients with cirrhosis during the COVID-19 pandemic
· Remdesivir GS-5734 a nucleoside analogue prodrug has inhibitory effects on pathogenic animal and human coronaviruses including severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 in vitro and inhibits Middle East respiratory syndrome coronavirus SARS-CoV-1 and SARS-CoV-2 replication in animal models, Methods, We did a randomised, double-blind, placebo-controlled, …
Care of patients with liver disease during the COVID-19
· Remdesivir • NUC/viral RNA polymerase inhibitor completed phase III for Ebola treatment • Inhibits SARS-CoV-2 in vitro 24 • Case reports with COVID-19 26 • No relevant drug-interactions expected 34 • No experience in liver cirrhosis but a NUC might be safer than other drug classes based on experience with NUCs in chronic hepatitis B
Use of remdesivir in the presence of elevated LFTs for the
The studies reporting outcomes with specific drugs like remdesivir hydroxychloroquine tocilizumab and convalescent plasma included a small patient number of cirrhosis patients and individual outcomes with drugs have not been reported3–5 7 Moreover, the safety and efficacy of these agents in COVID-19+cirrhosis patients is unclear and needs to be evaluated in future studies,
Usage experience of remdesivir for SARS-CoV-2 infection in
· Fichier PDF
Usage experience of remdesivir for SARS-CoV-2 infection in a patient with chronic cirrhosis of Child-Pugh class C J Antimicrob Chemother 2021 Mar 13;dkab076 …
Author : Takumi Umemura Kazuki Nishikawa Yoshikazu Mutoh Hajime Sasano Koji Kozaki, Tetsuya Yamada, Toshi
Usage experience of remdesivir for SARS-CoV-2 infection in
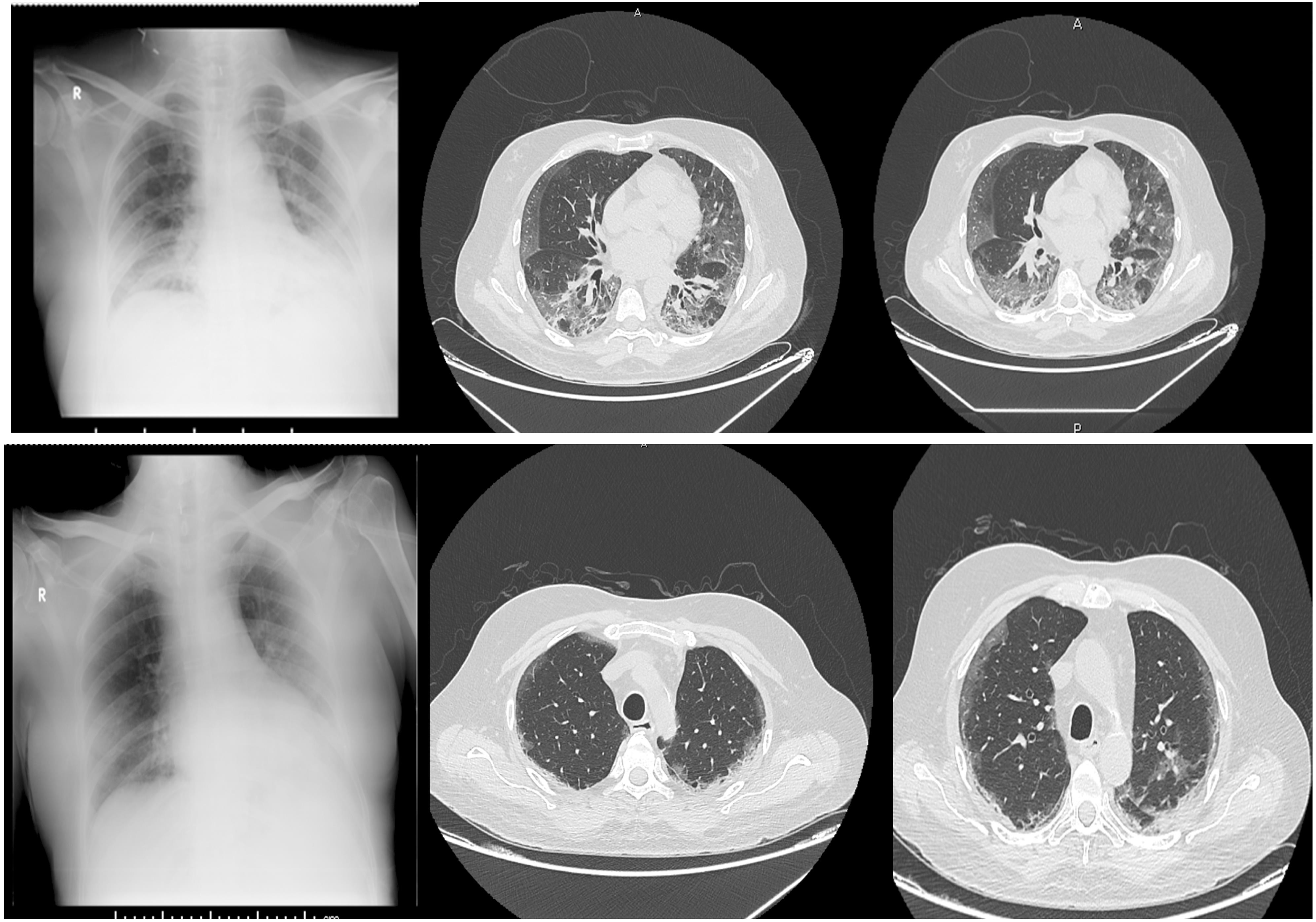
Usage experience of remdesivir for SARS-CoV-2 infection in
Remdesivir use may be appropriate in patients with elevated LFTs at baseline Intense monitoring is a necessity in these patients to avoid undue harm The choice to use remdesivir in this population should take into the consideration the risk and benefits of therapy However, further research is needed to develop a better understanding of the nature of hepatotoxicity risk and drug–drug
Remdesivir particularly improves the survival outcomes of patients under oxygen support compared with those of patients receiving placebo3 However clinical trials on remdesivir treatment excluded patients with hepatic impairment3–6 Currently little is known about the experience of remdesivir administration in COVID-19 patients with severe hepatic cirrhosis Herein we present the case of
Author : Takumi Umemura, Kazuki Nishikawa, Yoshikazu Mutoh, Hajime Sasano, Koji Kozaki, Tetsuya Yamada, Toshi
Usage experience of remdesivir for SARS-CoV-2 infection in
· Remdesivir may need to be discontinued if alanine transaminase ALT levels increase to >10 times the upper limit of normal and should be discontinued if an increase in ALT level and signs or symptoms of liver inflammation are observed, 3, Considerations in Patients With Renal Insufficiency, Each 100 mg vial of remdesivir lyophilized powder contains 3 g of sulfobutylether beta-cyclodextrin
· Cirrhosis is associated with inherent immune dysfunction and an altered gut-liver axis; During the early phase of the COVID-19 pandemic multiple drugs including antiviral agents eg, lopinavir/ritonavir remdesivir oseltamivir, ribavirin, antibiotics e,g,, macrolides, and corticosteroids, were prescribed, Many of these drugs are metabolized in the liver, and concerns about drug
Lieu : 8600 Rockville Pike, Bethesda, MD
Management of Remdesivir-Associated Acute Liver Failure
· 50 patients with cirrhosis and SARS-CoV-2 infection were studied with an overall 30-day mortality rate of 34% • Mortality was higher in patients with respiratory failure and in those with worsening liver function at COVID-19 diagnosis • 30-day mortality rates were higher in patients with cirrhosis and COVID-19 than in those with bacterial infections, • No major adverse events were